Describe How Changing the Particles Changed the Atom
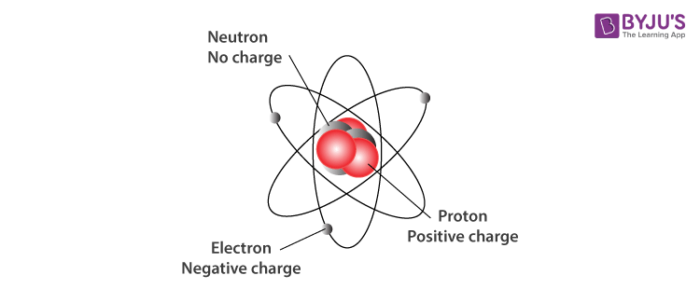
The nucleus which is in the center of the atom and contains protons and neutrons and the outer region of the atom which holds its electrons in orbit around the nucleus. The particles within an atom are bound together by powerful forces.
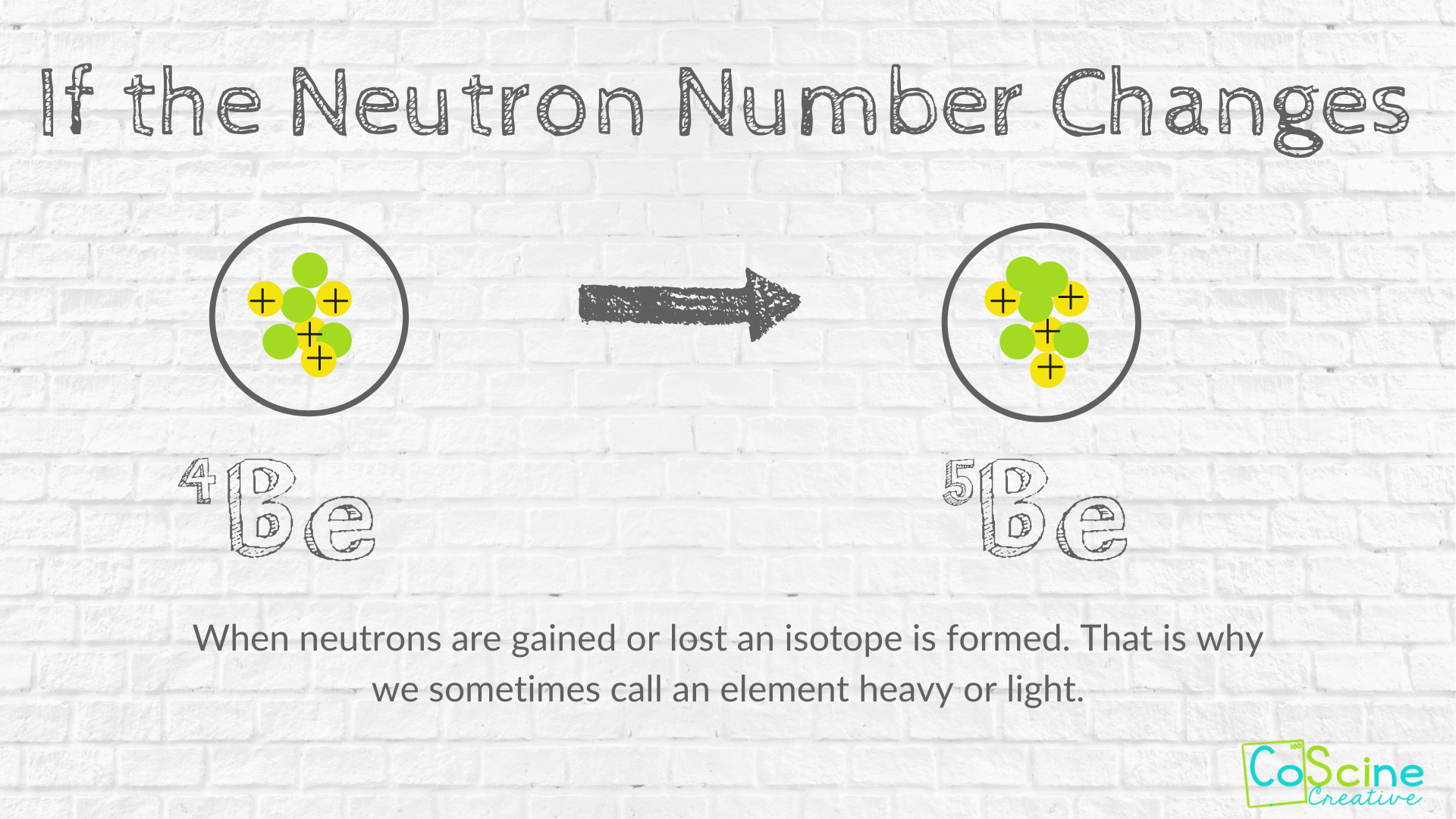
How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative
People also ask how was the atomic model developed.
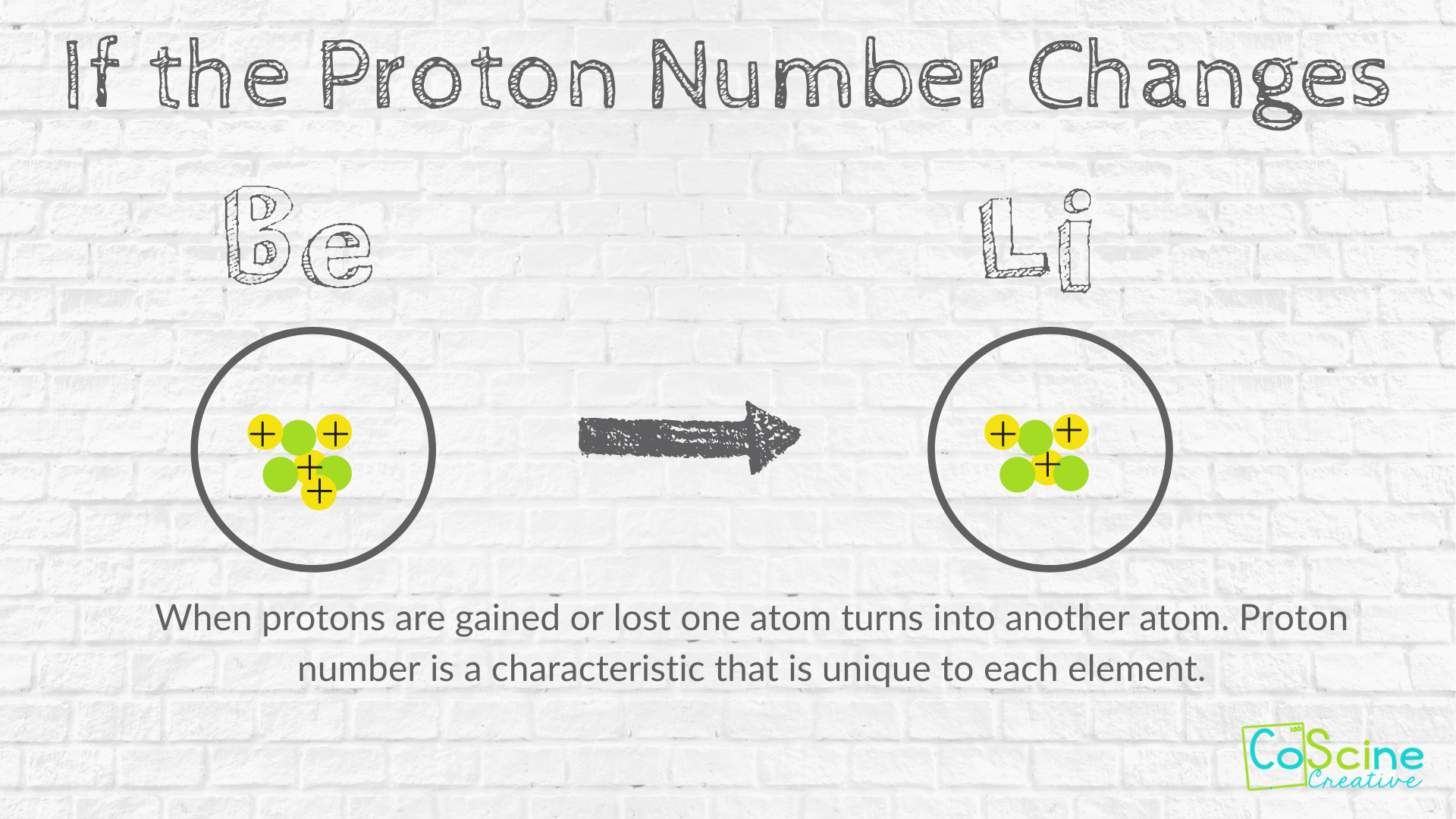
. Varying the number of electrons results in ions. During chemical changes particles do change with atoms or ions regrouping. These electrons may be removed from or gained by an atom to form ions.
In your answer include the work of Thomson Rutherford and Bohr and the Geiger-Marsden experiment. Ocean waves and sand erode a sea cave in a solid rock cliff. According to John dalton atomic theory atom is considered smallest unit which cannot be divided further.
The atom was described as a positively-charged sphere embedded with electrons. After the discovery of electron and proton the scientists started thinking of arranging these particles in an atom. Bonds links between atoms break and new ones form and energy is either given out or taken in.
How would you describe the Structure of an Atom. Answer 1 of 4. Describe how and why the atomic model has changed over time.
He concluded that all matter was made of tiny particles called atoms. If an atom loses an electron then it has more protons which makes it positively charged. The postulate of Daltons atomic theory that atom is indivisible particle and can be subdivided into smaller particles was later changed because atom can be divided into neutrons protons and electrons.
The scientist John Dalton carried out a series of experiments. Protons and neutrons have approximately the same mass about 167 10-24 grams which scientists define as one atomic mass unit amu or. Oct 11 2014.
Thomson was the first scientist to propose a model for the structure of atom. The 3 that seem to be being thought of here are among the most stable which is why they were discovered early on though the neutrino was in fact described if not observed before the neutron was and nobody is counting it in the t. In 1913 Neils Bohr a student of Rutherford s developed a new model of the atom.
Thomsons experiments changed the model of the atom to one containing positively and negatively charged particles dispersed throughout. During chemical changes particles do change with atoms or ions regrouping. The mass of the particles was very very small.
State the kind of change in matter in each example below. Over the last 100 years scientists have done investigations which show that atoms are made up of even smaller particles. An atom is composed of two regions.
The atom was imagined as a small indivisible ball similar to a very tiny ball. Electrons of different atoms come together to participate in chemical bonding. If an atom gains an electron then it has more electrons which gives it a negative charge.
The first model of the atom was developed by JJ Thomson in 1904 who thought that atoms were composed purely of negatively charged electrons. Occurring in the nucleus of the atom when it emits or absorbs rays or particles when the nucleus splits or joins with another. Many complex chemical phenomena occur around us and these are explained on the basis of the matter which is made up of molecules.
Electrons are the subatomic particles that revolve around the nucleus of an atom. In general electrons are easier to add or remove from an atom than a proton or neutron. Daltons Atomic Theory proposed that an atom is an indivisible particle.
The role of the scientist in the development of the quantum mechanical description of the atom of th. But later discovery of electron proton and neutron lead our knowledge further and now we know that atom is made. These electrons can be lost from or gained by an atom to form the ions.
The particles were deflected by magnetic as well as electrical field. A few points detailing the discovery and the properties of electrons are listed below. Start your trial now.
Bonds links between atoms break and new ones form and energy is either given out or taken in. This model was known as the plum pudding model. Matter is made of atoms which are tiny particles that cannot be created destroyed or divided.
But research findings of the last hundred years on the study of gases in particular and then of. Dome7w and 11 more users found this. The atomic model developed by scientists has changed over time as experimental evidence has improved our understanding of the structure of atoms.
Changing Models of the Atom. There are not 3 subatomic particles but many. The particles were about 2000 times lighter than the hydrogen ions.
Fallen snow compacts into glacier ice. JJThompson named the particle as corpuscle but. Equal number of both the electrons and protons constitute in the atoms of all the elements.
Thomson discovered the electron a negatively-charged particle. Electrons of several different atoms come together to participate in the chemical bonding. 100 1 rating Ans.
A solid rock decomposes into a different. In 1803 John Dalton presented his atomic theory based on three key ideas. Molecules in turn are made up of atoms.
Approved by eNotes Editorial Team Ask a Question. Solution for Describe how the particles change in position and movement as the two substances are mixed. Isotopes and ions of an atom with a constant number of protons are all variations of a single element.
Electrons are the subatomic particles which revolve around the nucleus of the atom. Due to the fact that electrons can transfer from one atom to another every atom has the possibility of becoming negatively or positively charged. The postulate of Daltons atomic theory that atom is indivisible particle and can be subdivided into smaller particles was later changed because atom can be divided into neutrons protons and electrons.
This theory was then disproved by Ernest Rutherford and the gold foil experiment in 1911 where Rutherford shot alpha particles at gold foil and noticed that. First week only 499.

Atomic Structure And Subatomic Particles Youtube

How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative
Comments
Post a Comment